Review of Paper 2:
The Proton-Pump Inhibitor Lansoprazole Enhances Amyloid Beta Production (Badiola et al.)
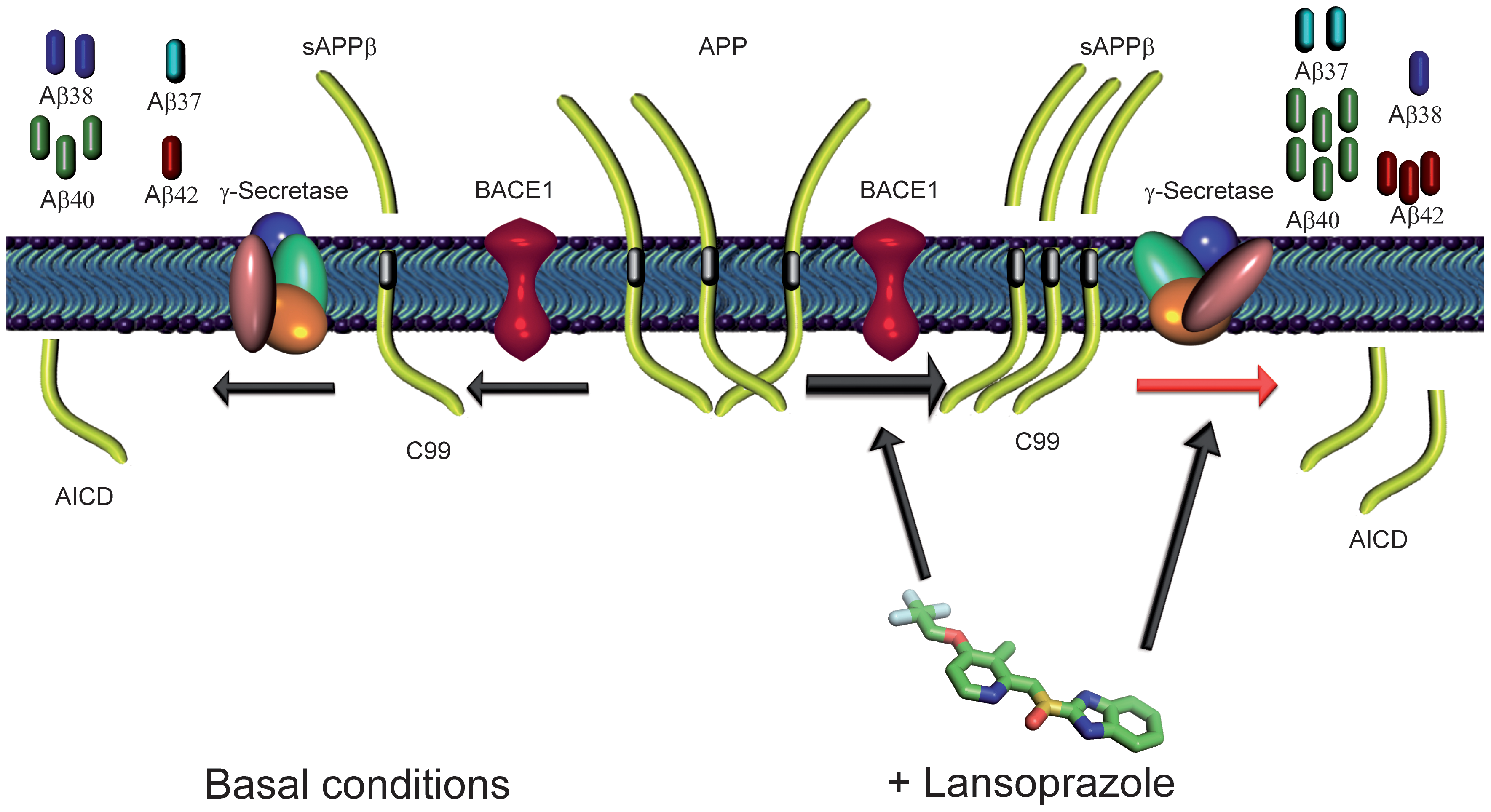
Image courtesy of Bandiola et al.
Summary & Opinion
In high school I was assigned to research the mechanisms and treatment of Alzheimer’s disease for a science presentation. At the time, the thing that astounded me the most was how little information was known about what exactly caused the disease and how to treat or prevent it. Therefore, I was very interested to analyze this article from a different lens, one that considers treatment options and clinical discoveries, and see if there were any more developments in the understanding of Alzheimer’s.
In general this paper looks to validate the accuracy of a system created by Badiola et al. that aims to screen and determine if commonly applied drugs, unrelated to Alzheimer’s, could be influencing the disease in either a positive or negative manner. There are currently only four drugs for AD on the market so determining any other drugs that may be also used for AD is critical. To understand what is being experimented a little background is needed.
Amyloid precursor protein is a protein found in the brain and when cleaved inappropriately by certain enzymes forms amyloid-β species that accumulate in the extracellular space. This accumulation, commonly known as amyloid plaques, lead to “oxidative and inflammatory damage” that then eventually “energy failure and synaptic dysfunction” within the neurons, which as been attributed to a major pathological component in AD (Badiola et al. 2013). Further studies have also linked the composition of these plaques, types of amyloid-β species (there are multiple cleavage sites on amyloid precursor protein) that are more soluble with the severity of symptoms and deleterious effects within the brain.
In this experiment, Badiola et al. looked at the clinical effects, both positive and negative, of commonly prescribed drugs determining what were the molecular effectors that the drugs utilized and the physiological response. By compiling all this information, they created a disease-related cell network, or a therapeutic performance mapping system (TPMS), that was used to predict the types of interactions or impacts unrelated drugs could have on the disease. Interestingly enough TPMS predicted that lansoprazole, a proton pump inhibitor commonly prescribed or peptic ulcer disease, played large role in the production of amyloid- β species. Therefore, the rest of the paper went on to experiment this prediction on mice with a goal to both see the effects of lansoprazole in AD as well as show that the system they created, TPMS, is able to accurately predict potential drug interactions in different diseases. The figures below present and describe their findings.
I think that the application of a top down systems biology approach to this issue is intelligent, compelling, and very good use of the labs time. We currently live in a world where drugs are frequently created and overprescribed without thought about the potential effects these drugs could have on other organ systems. Even though I do not know much about the screening process for drugs before they go on the market, I would guess that there are many unknown long-term effects that drugs have on all untargeted biological systems. However, individually screening and testing for all the effects of drugs is impossible and therefore the creation of a system that could potentially predict hazards is critical in our society. Furthermore, we have seen in our class that drugs prescribed for certain diseases can also effectively used for others. Therefore, systems like TPMS could also be used to redirect and reintroduce drugs already created for different diseases. In this way I commend Badiola et al. for their innovation.
In general the article was very well cited (57 sources), adding validity to their findings as it is important in systems biology to collect all the information previously published and piece it together to form the bigger picture. The figures themselves were clear, contained standard error bars, and generally used large sample sizes. However I had some individual problems with the certain graphs and blots, which are expressed below. In general, I did not think that any of the Western blots clearly verified the results that Bandiola et al. was trying to confirm and no reference scale bar was ever included. However, I thought overall the paper did a good job at showing their system had credibility. Furthermore, the paper never claimed to prove that lansoprazole had effects on AD but rather to present the data for further investigation saying in their closing line, “These results can serve as a catalyst for further studies in order to evaluate whether the treatment with PPIs may have an impact on AD pathology” (Bandiola et al. 2013). With this mind, I think that this paper was credible and innovative.
Analysis of Figures
Figure 1
In general these two panels are showing the results of Bandiola et al.’s first round of experiments. In the following the exposed hamster ovary cells expressing the APP protein to different PPI’s at different concentrations. After 24 hours of exposure, they measured the amount of Aβ40 and Aβ42 produced. The lab had a vehicle control and a negative control (DAPT), which were cells expressing an enzyme that inhibited the Υ-secretase or protein responsible for cleaving APP. In general much more evidence but this showed a good starting place.
A. These two bar graphs display the percentage of Aβ40 and Aβ42 with increasing lansoprazole concentration. The data was normalized to the vehicle control and the negative control accurately shows almost no production of both types of amyloid produtcts. The results showed that cells treated with over 10μm produced double the amount of Aβ40. Further there was a drug dependent rate of increase in Aβ42 production between 10μm and 50 μm. All bars had standard deviation but only a few with a noted p value of either <.05 and <.01 especially in the graph showing Aβ40 amounts.
B. After the results shown above, Bandiola’s team went on to test the effects of other commonly prescribed proton pump inhibitors (omeprazole, pantoprazole, esomprazole) using the same experimental design and graphical display. An increase in both Aβ40 and Aβ42 was observed for all the drugs but not to the same degree as lansoprazole. Again the Aβ40 was less uniform and contained no p values and in my opinion standard deviation bars were too large and overlapping to show significance. However, Aβ42 graph showed the dosage dependent rate of production and had significant p values.
Figure 2
To follow up the results above, the research team looked to confirm and explore the types of amyloid species produced and how lansoprazole contributed to their formation. They therefore too the media that was exposed to the highest concentration and immunoprecipitated it and used mass spectrometry analysis. The following panels display the evidence they propose suggests that lansoprazole works as an invers GSM, or a molecule that modulates Υ-secretase altering where it cleaves APP resulting in higher levels of Aβ42 and lower levels of Aβ38.
A. Panel A shows the results of the mass spectrometry. They compared the lansoprazole sample to the vehicle control as well as the negative DAPT control (which accurately showed no amyloid-β products). I would classify this as good evidence as the peaks are clear and I am able to see a definitive increase in Aβ42 and decrease of Aβ38 between the vehicle control and lansoprozole sample. However, the Aβ40 peak does not look that different and I would not be willing to say that this is evidence that Aβ40 species is increased in the lansoprazole sample.
B. To confirm the results of the mass spectrometry, Bandiola et al. used a Western blot to show the change in protein amount. I have the most trouble trusting this figure for a number of reasons. First, there is no reference cale bar. Second, the results are not clear at all and rather smudged considering that the amyloid species are all located right next to each other on the gel. I do not see an distinct increase in Aβ40 between the vehicle and lansoprazole. Furthermore, the bands for both Aβ38 and Aβ42 are very faint and could be due to smudging from the large Aβ40. I am able to see a very slight difference but I would not be the first one to validate this Western blot.
C. To further test their theory that lansoprazole was working as an inverse GSM they looked to see if it would counteract the effects of a known GSM, R-flurbiprofen, which decreases Aβ42 and increases Aβ38 (surprise right!). The bar graph showed that exposure to both lansoprazole did counteract R-flubiprofen and increased levels of Aβ42 compared to the vehicle control were observed. This particular graph showed significant p values across all samples, which was nice to see. However, there was no western blot performed, which would have added credibility.
D. The iGSM model accounted for the change in Aβ42 and Aβ38 products but not the increase of Aβ40 and Aβ37. There are two pathways that APP is cleaved by, non amyloidogenic (Υ-secretase and α-secretase) or the amyloid pathway (Υ-secretase and BACE1). In this panel they measured the amount of APP and BACE1 in the vehicle control and lansoprazole treated cells looking to see if the increase in products could simply be do to increased amount substrate(APP) or cleaving enzyme (BACE1) . They used actin as a control and the results do clearly show that there was no change in levels of either BACE1 or APP. Again, a reference scale bare would have been nice.
E. Badiola et al. then continued to experiment the types of cleavage products to try to determine which cleavage pathway (mentioned in Panel D) the lansoprazole was affecting. They therefore showed the increase of SAPPβ, cleavage product of BACE1, in a Western blot implying an increased activity of BACE1. Furthermore, sAPPα, cleavage product of α-secretase, was unchanged between the two samples implying that lansoprazole was affecting the amyloid pathway.
Figure 3
Until this point, all experiments had been done using cells, not a living organism. Furthermore, all of these results wouldn’t matter if lansoprazole could not cross the blood brain barrier and actually effect cells in the brain. Therefore, Bandiola et al. experimented on mice, injecting lansoprazole (a 20 mg/kg or a 100 mg/kg dosage) in both wild type and an AD triple-transgenic mice for five days and then examined amounts of Aβ40 and Aβ42 species. I think that this experiment was crucial to their results as it showed increases of Aβ40 and Aβ42 species in a living species instead of just cells. The data did not display the exact same trends as seen in cells (Aβ42 production did not increase as drastically), but a clear increase can be seen. Furthermore, they referenced the fact that the amount of lansoprazole injected was comparable (based on body mass differences) and even less than the amount humans ingested.
A. This graph is similar to all the other bar graphs observed in the paper displaying the increase of Aβ40 and Aβ42 species in wild type mice injected with 100kg/mg of lansoprazole versus the vehicle control. The data was normalized and only one bar shows significant p value. However, there was a large sample size and a clear increase can be seen for both Aβ40 and Aβ42. The effects are not as amplified as seen in the cells tested before which is to be expected in a living organism.
B. This graph shows the amount of Aβ40 and Aβ42 in 3xTg-AD mice injected with the vehicle, 20 mg/Kg or 100 mg/kg. The effect of lansoprazole is amplified and more apparent in these mice especially in regards to Aβ40 production, which rises in a dosage dependent manner. The amount Aβ42 also increases but not as drastically and no significant p values are added.
Figure 4
This final figure ties in all the results found above and Bandiola et al. present a hypothesized model that shows how lansoprazole may be interacting with APP to create these different amyloid species. They clearly state that the model is a merely a hypothesis and needs further testing. In this model they say that lansoprazole may both increase BACE1 activity ultimately leading to increased Aβ products and shifts the Υ-secretase cleavage cite leading to increased Aβ42 and decreased levels of Aβ38. The synthesis of ideas is important but I do not know if the diagram clearly displays what they are trying to say. I wish that they separated basal conditions from lansoprazole treated ones and explained what the green lines were since at first glance all I could gather was that it was a pretty picture.
References
1) Badiola N, Alcade V, Pujol A, Münter L-M, Multhaup G, et al. (2013) The Proton-Pump Inhibitor Lansoprazole Enhances Amyloid Beta Production. PLoS ONE 8(3): 1-8. Acessed 8 Apr. 2013. <http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0058837>
Email Questions or Comments to kagwathmey@davidson.edu
© Copyright 2013 Department of Biology, Davidson College, Davidson, NC 28035